This post was originally published on this site Breast cancer patients in the U.S. who are receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2-), and have mutated PIK3CA can now be treated with Novartis‘ Piqray (alpelisib) in combination with Faslodex (fulvestrant). The U.S. Food and Drug Administration (FDA) has approved the use of this…
Author: Chris
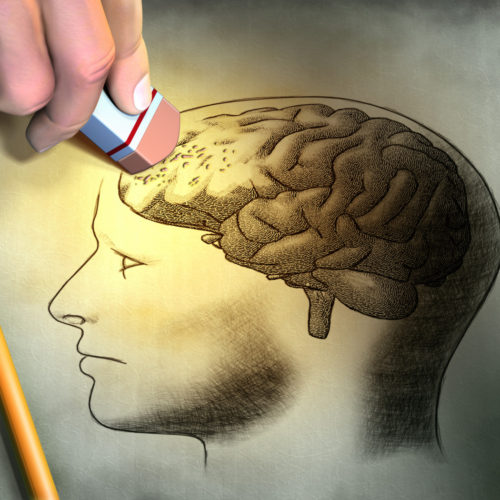
Early Brain Alterations May Occur Decades Before Onset of First Symptoms, Researchers Say
This post was originally published on this site Scientists have found a series of brain alterations associated with Alzheimer’s disease that occur decades before the onset of the first symptoms. Identifying these biological and anatomical brain alterations in people at high-risk for the disease may help them receive earlier therapeutic interventions, the researchers said. The study,…
RB1 Mutations Linked to Worse Outcomes in Prostate Cancer Patients, Study Finds
This post was originally published on this site Men whose tumors contain mutations in the retinoblastoma (RB1) gene have higher risks of prostate cancer relapse and mortality while receiving first-line conventional anti-cancer therapies, a study says. According to researchers, genetic screenings aimed at identifying patients whose tumors may contain such genetic alterations may be crucial…
Grant Lets Researchers Study OC Patients’ Responses to PARP Inhibitors
This post was originally published on this site Researchers at Michigan State University (MSU) were granted $50,000 to investigate why only a fraction of ovarian cancer patients respond to treatment with PARP inhibitors. The grant, awarded by the Michigan Ovarian Cancer Alliance, will allow the team to better understand the biological mechanisms underlying patients’ response…
Open Enrollment for Phase 1 Trial Testing CS1-CAR T-Cell Therapy for Hard-to-Treat Myeloma
This post was originally published on this site A Phase 1 clinical trial is recruiting patients with hard-to treat or relapsed multiple myeloma to evaluate Mustang Bio’s investigational autologous (patient-derived) CS1-chimeric antigen receptor (CAR) T-cell therapy. The trial (NCT03710421), designed to assess the therapy’s safety and tolerability, is being conducted at the City of Hope…
Preclinical Study Uncovers Resistance Mechanisms to Lymphoma Therapy and Potential Way to Overcome Them
This post was originally published on this site Lymphoma cells can become resistant to therapy as they acquire novel genetic mutations, but a new study has found that they can also become resistant as they reprogram which genes are turned “on” or “off.” This particular resistance mechanism was seen in B-cell lymphomas in response to…
Cosibelimab Shows Signs of Anti-cancer Activity Against Multiple Tumor Types in Phase 1 Trial
This post was originally published on this site Treatment with Checkpoint Therapeutics’ investigational immunotherapy Cosibelimab supports a positive anti-cancer response in approximately 28% of patients with advanced solid tumors, interim results from an ongoing Phase 1 trial show. In particular, the therapy showed greater potential to treat those with non-small-cell lung carcinoma and cutaneous squamous cell…
The Blonde in the Waiting Room
This post was originally published on this site A young woman weeped. Pretty blond hair swung in front of her face, but it didn’t hide her angst. From where I sat, I could feel the panic running though her body. It cut through the room like a chilly, wet breeze and crept into my lungs.…
The Death of Denial
This post was originally published on this site I was helping Mom in the bathroom when suddenly she slumped over, unable to hold herself upright. Her hospice nurse was scheduled to come later that day but had previously offered to come sooner if needed, so I immediately contacted her. As soon as she saw Mom, she…
Agency Unveils RaDaR to Help Patient Groups Develop Rare Disease Registries
This post was originally published on this site RaDaR, the catchy new name for the U.S. government-run Rare Diseases Registry Program, aims to help patient advocacy groups with limited resources build their own disease registries. The site was developed by the National Center for Advancing Translational Sciences (NCATS), a division of the National Institutes of…