With help from actress Kristen Bell, the Prostate Cancer Foundation (PCF) has opened its 4th annual TRUE Love contest for entries that pay tribute to family members and caregivers of men living with prostate cancer. The organization is seeking caregiving narratives that show love, care, and honor for prostate cancer patients to raise awareness about…
Author: Chris
ENB-003 with Keytruda Shows Promise Against Advanced Solid Tumors
ENB Therapeutics’ lead novel anti-cancer therapy, ENB-003, was found to be safe and showed promising anti-tumor activity, when given in combination with Keytruda (pembrolizumab), to adults with different types of advanced solid malignancies, including ovarian cancer and melanoma. These are the early findings of an open-label Phase 1/2 trial (NCT04205227) that has recently finished dosing…
Trial Testing AL101 in TNBC Doses First Patient
The first participant has been dosed in the Phase 2 clinical trial TENACITY, which is evaluating Ayala Pharmaceuticals‘ investigational therapy AL101 as a treatment for Notch-activated recurrent or metastatic triple negative breast cancer (TNBC). AL101 is an investigational small molecule that inhibits Notch proteins. This family of cellular proteins plays important roles in governing cell…
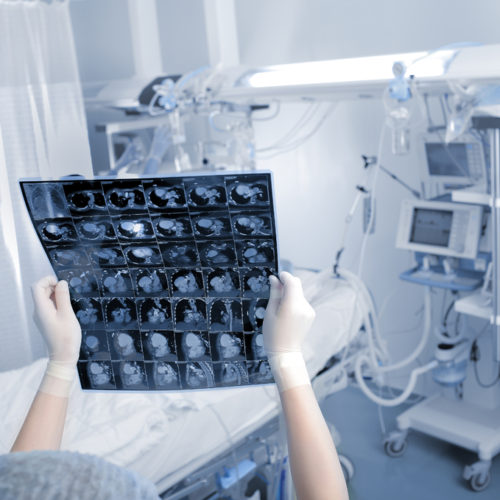
New AI Tool May Better Predict Relapse After Surgery
Researchers have developed an artificial intelligence (AI) tool that shows the potential to predict prostate cancer aggressiveness and the likelihood of post-surgery relapse more accurately than standard methods can. Called RadClip, for radiomic-clinicopathologic nomogram, the tool was developed by a team from the Center for Computational Imaging and Personalized Diagnostics (CCIPC) at Case Western Reserve…
FDA Clears Way for Clinical Trial of HDP-101
Heidelberg Pharma has been cleared in the U.S. to begin a clinical trial testing its investigational therapy HDP-101 for the treatment of multiple myeloma. The U.S. Food and Drug Administration (FDA) allowed the start of a Phase 1/2a trial following the submission of an investigational new drug (IND) application. The company expects to enroll the…
CAR T-cell Therapy Breyanzi Approved for Large B-cell Lymphomas
The U.S. Food and Drug Administration (FDA) has approved the CAR T-cell therapy Breyanzi (lisocabtagene maraleucel) for treating adults with relapsed or refractory large B-cell lymphoma (LBCL) who have had two or more lines of systemic therapy. The approval covers several types of LBCL: diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising…
Alzheimer’s Association Webinar Tackles Care Disparities for Blacks in US
Older Blacks in the U.S. are twice as likely to develop Alzheimer’s disease (AD) as whites of a similar age, and they’re less likely to be diagnosed. Such a health disparity is why the Alzheimer’s Association is presenting a webinar on Feb. 18, marking Black History Month. Called “Creating a Path Forward: Reaching Health Equity…
€15M EU Project Seeks to Improve Personalized Medicine Options
The European Union is providing nearly €15 million ($18.1 million) over five years to improve personalized medicine options for people with drug-resistant high-grade serous ovarian cancer. The DECIDER project, funded by the EU’s Horizon 2020 research and innovation program, will apply artificial intelligence methods to develop new diagnostic tools to identify — earlier and more…
Under-the-skin Velcade Found to Work Better in Farydak Triple Combo
Using an under-the-skin formulation of Velcade (bortezomib), in combination with Farydak (panobinostat) and oral dexamethasone, leads to a better safety profile in people with relapsed or refractory (resistant) multiple myeloma than does the therapy’s original into-the-vein version, data show. The triple combination treatment with under-the-skin Velcade also was found to induce longer responses than the…
Keytruda-Yervoy Combo of No Benefit in Advanced NSCLC, Trial Data Show
First-line treatment with a combination of Yervoy (ipilimumab) and Keytruda (pembrolizumab) failed to prolong survival and delay disease progression in patients with advanced non-small cell lung cancer (NSCLC) compared with Keytruda alone, according to data from a Phase 3 trial. The KEYNOTE-598 (NCT03302234) trial involved patients whose tumors produced high levels of PD-L1 and harbored…