This post was originally published on this site The U.S. Food and Drug Administration (FDA) has approved — with conditions — Xpovio (selinexor) tablets, in combination with the corticosteroid dexamethasone, for the treatment of adults with relapsed or refractory multiple myeloma who received at least four prior therapies and failed to respond to treatment. The…
Category: <span>Blog</span>
Workplace Hazards Account for 30% of Sarcoidosis Cases, Study Suggests
This post was originally published on this site Work-related hazards account for nearly 1 in 3 cases of sarcoidosis, and for at least 1 in 10 cases of other respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and bronchitis, a new analysis suggests. The analyss, “The Occupational Burden of Nonmalignant Respiratory Diseases. An Official…
Puma Applies to FDA to Extend Nerlynx’s Use to HER2-Positive Metastatic Breast Cancer
This post was originally published on this site Puma Biotechnology is seeking to extend the use of its breast cancer medicine Nerlynx (neratinib), in combination with capecitabine, to treat women with HER2-positive breast cancer whose disease has spread to other parts of the body, and is metastatic. In a supplemental New Drug Application (sNDA) filed…
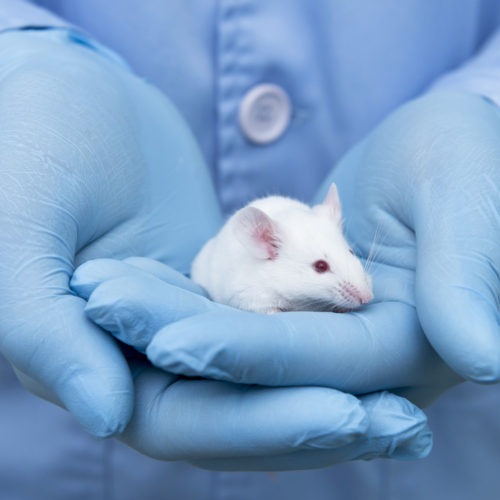
Seizures May Reduce Brain Cell Production, Result in Poor Spatial Discrimination, Mouse Study Suggests
This post was originally published on this site Spontaneous seizures reduce the production of new brain cells, and result in poor spatial discrimination — a common occurrence in people with Alzheimer’s disease, according to a mouse study. The study, “Early Seizure Activity Accelerates Depletion of Hippocampal Neural Stem Cells and Impairs Spatial Discrimination in an…
World’s First Alport Stamp Is Macedonian Mom’s Latest Win for Rare Disease Patients
This post was originally published on this site It wasn’t until Gordana Loleska’s son David was 14 years old that doctors in their native North Macedonia diagnosed his kidney, vision, and hearing problems as Alport syndrome. Although she had known for years that something was wrong, the news that David would battle a lifelong rare…
Adding Xtandi to Xofigo Does Not Raise Risk of Bone Issues in CRPC, Study Says
This post was originally published on this site Metastatic castration-resistant prostate cancer (CRPC) patients who receive Xtandi (enzalutamide) plus Xofigo (radium-223) in a real-world setting do not experience more fractures or bone-related events than those given Xofigo alone, an observational study shows. This has been a common concern for metastatic CRPC patients, among whom Xofigo…
New Blood Test May Improve Ovarian Cancer Diagnosis, Study Suggests
This post was originally published on this site A blood test that examines a panel of 11 specific proteins could help distinguish between benign tumors and malignant ovarian cancer, reducing the need for unnecessary surgeries and leading to an earlier diagnosis, a study shows. Conducted by researchers at Uppsala University and Sahlgrenska Academy at the…
Scientists Find ‘On/off Switch’ of Malignant MM Cancer Cells, May Lead to New Therapies, Study Says
This post was originally published on this site Scientists discovered a set of genes that work as an “on/off switch” for malignant multiple myeloma cancer cells, which may shed light into new therapeutic targets for the disease, and for other types of cancers with a higher tendency to spread to bones. The findings of the…
Imfinzi Combo Therapy Extends Survival of Advanced SCLC Patients, Phase 3 Trial Shows
This post was originally published on this site Adding Imfinzi (durvalumab) to standard first-line chemotherapy led to a clinically meaningful extension in the overall survival of patients with advanced small cell lung cancer (SCLC), according to results of a Phase 3 clinical trial. The open-label, global CASPIAN trial (NCT03043872) is testing AstraZeneca’s Imfinzi combined with…
CHMP Recommends Tecentriq-Abraxane Combination to Treat Some Triple-negative Breast Cancers in Europe
This post was originally published on this site The Committee for Medicinal Products for Human Use (CHMP), an arm of the European Medicines Agency , has recommended the approval of Tecentriq (atezolizumab) in combination with the chemotherapy Abraxane (nab-paclitaxel) as a first-line treatment for some advanced triple-negative breast cancers (TNBC). Specifically, the recommendation is for adult patients with…