This post was originally published on this site The National Institute for Health and Care Excellence (NICE) in England has recommended treatment with Revlimid (lenalidomide) and dexamethasone for multiple myeloma patients not eligible for a stem cell transplant or thalidomide therapy. “This decision is fantastic news for the myeloma patients who will be able to access…
Category: Cancer
Japanese Researchers Use Hyperion Imaging Tool to Advance Treatment Discovery for Colorectal Cancer
This post was originally published on this site The National Cancer Center (NCC) in Japan is advancing its research into novel immunotherapies for colorectal cancer by using the Hyperion Imaging System, a powerful tool that enables simultaneous visualization of dozens of markers in a sample of tissue. The system, by Fluidigm, combines CyTOF technology with…
Treatment Intensity Should Be Personalized in Women with HER2-positive Breast Cancer, Study Says
This post was originally published on this site Personalized treatment intensity is crucial to the success of less toxic and demanding therapies for women with HER2-positive breast cancer, according to a spokesperson from the European Society for Medical Oncology (ESMO). The remarks by Carmen Criscitiello, MD, PhD, accompanied updated results from the PERNETTA trial (EudraCT…
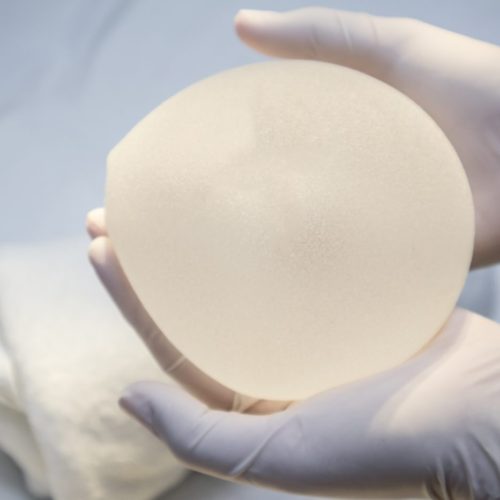
FDA Won’t Ban Sales of Breast Implants Linked to Lymphoma, But Considers Black Box Warning
This post was originally published on this site In announcing that breast implants linked to anaplastic large-cell lymphoma (ALCL) can continue to be sold in the United States, the U.S. Food and Drug Administration (FDA) proposed assurances that the potential risks of such implants are better known. In its recent statement, the agency said it would…
Ampligen Showing Promise for Recurrent Ovarian Cancer in Phase 1/2 Trial, Phase 2 Study Now Recruiting
This post was originally published on this site Hemispherx Biopharma‘s experimental therapy Ampligen (rintatolimod) can change the tumor microenvironment to increase the amount of infiltrating T-cells, an approach that is showing promise in a Phase 1/2 clinical trial for recurrent ovarian cancer, according to the company. Funded in part by an Ovarian Cancer Specialized Program…
Therapy for Heavily Treated Multiple Myeloma Gets Fast Track Status from FDA
This post was originally published on this site Cellectar Biosciences’ candidate CLR 131 received Fast Track designation by the U.S. Food and Drug Administration (FDA) for the treatment of multiple myeloma patients who have failed at least four prior lines of therapy, the company announced. The designation is intended to speed the therapy’s development and…
Young Women More Likely to Face Aggressive BC Types, But Proper Treatment Improves Outcomes
This post was originally published on this site Young women are more likely than older ones to be diagnosed with more aggressive forms of breast cancer, but their outcomes can be good if they follow recommended treatments. That is the take-home messages of two studies presented at the recent ESMO Breast Cancer Congress 2019, held…
Revlimid and Imnovid Triple Combos Approved in EU for Adults with Multiple Myeloma
This post was originally published on this site The European Commission has approved two combination therapies based on Celgene‘s Revlimid (lenalidomide) and Imnovid (pomalidomide), added to Velcade (bortezomib) and dexamethasone, for the treatment of some adult patients with multiple myeloma. Revlimid, combined with Velcade and dexamethasone (RVd), is now indicated for the treatment of newly diagnosed, untreated adults…
FDA Approves Venclexta-Gazyva Combo for Untreated CLL/SLL Patients
This post was originally published on this site The U.S. Food and Drug Administration (FDA) has approved the double combination therapy of Venclexta (venetoclax) and Gazyva (obinutuzumab) for the treatment of adults with newly diagnosed chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Venclexta is a potent selective inhibitor of the B-cell lymphoma-2 (BCL-2)…
First Patients Dosed in iTeos’ Experimental A2A Receptor Antagonist Trial for Solid Tumors
This post was originally published on this site iTeos Therapeutics’ Phase 1/1b clinical trial testing its experimental A2A receptor antagonist, EOS100850, has dosed the first group of patients with advanced solid tumors, the company announced. “I am tremendously excited to announce that patient dosing in our innovative, adaptive Phase 1/1b trial is now underway with…