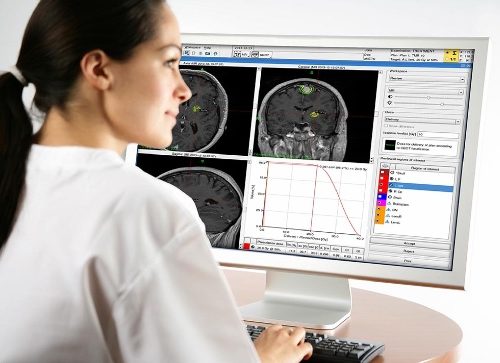
This post was originally published on this site The FDA published the approval of the first focused ultrasound device for the treatment of essential tremor in people who did not respond to treatment. Magnetic resonance (MR) images are taken by ExAblate Neuro during application of focused ultrasound to kill the brain cells which are considered…
Category: <span>Cancer</span>
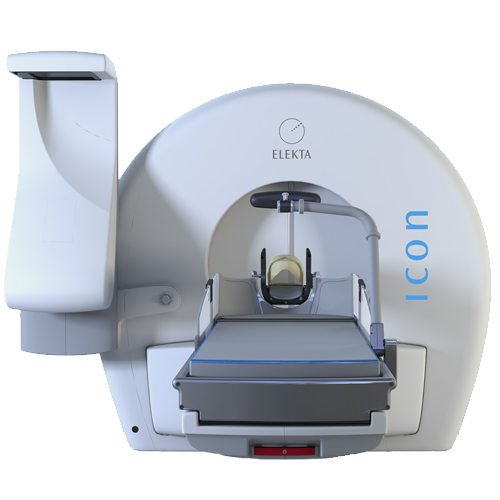
1 Million Patients Treated with Leksell Gamma Knife Surgery — a Milestone for Elekta
This post was originally published on this site World neurosurgery leaders gathered in Amsterdam May 15-19 for the 18th International Leksell Gamma Knife Society Meeting. The annual conference is dedicated to Gamma Knife surgery in the treatment of intracranial disorders, and covers topics such as radiosurgery, benign tumors, vascular disorders, malignant tumors, ophthalmological disorders, functional…
Opdivo-Yervoy Combo Shows Signs of Activity in CRPC Patients with Metastases
This post was originally published on this site A combination of Bristol-Myers Squibb’s immune checkpoint inhibitors Opdivo (nivolumab) and Yervoy (ipilimumab) reduces tumor burden in castration-resistant prostate cancer patients whose tumors have spread, interim Phase 2 results show. Findings from the CheckMate 650 trial (NCT02985957) were recently shared at the 2019 Genitourinary Cancers Symposium in San Francisco in a presentation…
Xtandi, Zytiga Better for African-Americans Than Whites With Advanced Prostate Cancer, Research Finds
This post was originally published on this site African-Americans with metastatic castration-resistant prostate cancer live longer after treatment with the androgen inhibitors Zytiga (abiraterone acetate) or Xtandi (enzalutamide) than white men, a new study reports. Historically, African-Americans diagnosed with prostate cancer have had worse survival outcomes than whites. The reasons for this are complex and…
Leap’s DKN-01 Enters Testing in Metastatic Castration-resistant Prostate Cancers
This post was originally published on this site Leap Therapeutics is launching a Phase 1/2 trial to study its lead candidate DKN-01, alone or in combination with chemotherapy, for the treatment of metastatic castration-resistant prostate cancer (CRPC) patients whose tumors have elevated Dickkopf-1 (DKK1) levels. The trial (NCT03837353), led by David Wise, MD, PhD, will be conducted…
Physicians’ Education Resource to Host 12th Annual Prostate Cancer Congress
This post was originally published on this site The 12th annual New York GU: Annual Interdisciplinary Prostate Cancer Congress and Other Genitourinary Malignancies will focus on essential clinical topics in disease management. The meeting, hosted by Physicians’ Education Resource, a top resource for continuing medical education, will be March 15-16 in New York. It will be…
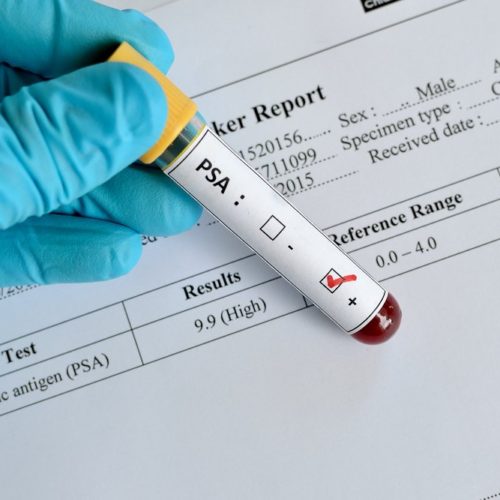
PSA Testing Cuts Deaths, Shows Value of Long-Term Screening
This post was originally published on this site A blood test measuring the levels of PSA — a well-known marker of prostate cancer — cuts deaths from the disease by nearly 30%, the longest screening study on prostate cancer shows. The study, “Improving Prostate Cancer Screening: 22-Year Follow-up in a Randomized Trial,” followed 20,000 men…
FPA150 Trial Begins Dosing Ovarian, Breast, Endometrial Cancer Patients
This post was originally published on this site Five Prime Therapeutics’ ongoing Phase 1 clinical trial has advanced to its Phase 1b expansion portion, in which researchers have begun giving breast, ovarian, and endometrial cancer patients an optimal dose of the company’s immune checkpoint inhibitor, FPA150. “We are very pleased with the progress of our FPA150 clinical…
Phase 2 Trial Completes Dosing First OC Patient with Aivita’s Cell Therapy
This post was originally published on this site A Phase 2 trial exploring Aivita Biomedical’s cell therapy AVOVA-1 for ovarian cancer has treated its first patient and continues to recruit participants across eight sites in the United States. So far, 11 patients have been included in the study, including one who received the planned eight treatment sessions, under…
UPMC Trial to Explore Ampligen Combo for Recurrent Ovarian Cancer
This post was originally published on this site A new investigator-sponsored clinical trial is recruiting women with recurrent, platinum-sensitive ovarian cancer to study a combination of Hemispherx Biopharma’s investigational product Ampligen (rintatolimod) plus chemo-immunotherapy, the company announced. The Phase 1/2 trial (NCT03734692) is being conducted at the UPMC Hillman Cancer Center in Pittsburgh, Pennsylvania, under the supervision of…