The National Ovarian Cancer Coalition (NOCC) is hosting its annual Together in Teal — No Boundaries fundraiser as a nationwide virtual celebration on Sept. 26, it announced in a press release. Presented during National Ovarian Cancer Awareness Month, the event raises money to support early disease awareness, quality of life for survivors, scientific research, and community…
Category: Cancer
Melflufen Combo Earns FDA Priority Review for Triple-refractory Myeloma
The U.S. Food and Drug Administration (FDA) has granted priority review to Oncopeptide’s application seeking accelerated approval of melflufen (melphalan flufenamide) plus dexamethasone for people with relapsed or refractory multiple myeloma (RRMM). The new drug application was submitted in July and based on promising data from the HORIZON Phase 2 trial (NCT02963493). It is specific for people with triple-refractory disease —…
FDA Rejects Keytruda-Lenvima Combo as First-line Treatment for Liver Cancer
The U.S. Food and Drug Administration (FDA) has issued a complete response letter stating that it will not approve the combination of Keytruda (pembrolizumab) plus Lenvima (lenvatinib) as a first-line treatment for people with advanced unresectable hepatocellular carcinoma (HCC). According to a press release, the decision was made on the basis that there isn’t evidence this treatment combination…
‘Eat It to Beat It’ Challenge to Honor Prostate Cancer Awareness Month
To honor Prostate Cancer Awareness Month this September, the Prostate Cancer Foundation (PCF) has launched its “Eat It to Beat It” challenge with the endorsement of celebrity ambassador Harry Lennix, an actor currently starring in NBC’s The Blacklist. The challenge asks participants to eat 30 healthy foods during the month to raise awareness about how…
Research Alliance Hopes to Raise $200K During Awareness Month
The Ovarian Cancer Research Alliance (OCRA) is hoping to raise $200,000 in this year’s awareness month campaign to help fund research and support those with the disease, the organization announced. OCRA, the largest non-government funder of ovarian cancer research, is urging people worldwide to make single or monthly donations, which can be dedicated in memory…
FDA Reviewing Oral Paclitaxel Combo as Metastatic Breast Cancer Treatment
The U.S. Food and Drug Administration (FDA) is reviewing a new drug application from Athenex seeking approval of oral paclitaxel and encequidar (Oral Paclitaxel) for the treatment of metastatic breast cancer. The application has been granted priority review, and the FDA is expected to make a decision no later than Feb. 28, 2021. The FDA,…
Trial Begins Dosing of ‘Off-the-shelf’ CAR T-cell Therapy for Multiple Myeloma
The first patient has been dosed in a Phase 1/2a trial investigating Precision BioSciences‘ new CAR T-cell therapy candidate, PBCAR269A, for people with relapsed or refractory multiple myeloma. The dose-escalation trial (NCT04171843) is expected to recruit 48 participants at five clinical sites in the U.S. Enrollment is now active at City of Hope, in California, and…
Imfinzi Approved in EU for Extensive-stage Small Cell Lung Cancer
The European Commission has approved a combination of Imfinzi (durvalumab) plus standard chemotherapy as a first-line treatment for people with extensive-stage small cell lung cancer (SCLC). Findings from the CASPIAN Phase 3 trial (NCT03043872) supported the decision. There, newly diagnosed patients with extensive-stage (widely spread) SCLC who received the combination lived significantly longer than those…
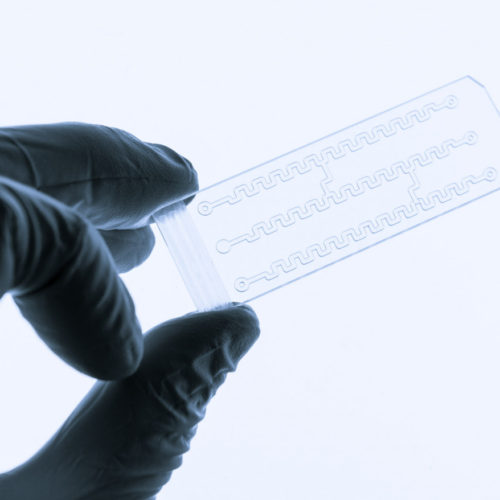
Organ-on-a-chip Device Shows How Cancer Cells Interact With Platelets to Drive Metastasis
To communicate with platelets and start the process of cancer spread, ovarian cancer cells are able to break the barriers of blood vessels and let platelets inside the tumor environment, according to a study that used microfluidic organ-in-a-chip technology. Atorvastatin, a medication belonging to cholesterol-lowering statins, is able to maintain blood vessel integrity and prevent…
Cilta-cel, CAR T-cell Treatment, Named Breakthrough Therapy in China
Legend Biotech and Janssen’s investigational CAR T-cell therapy ciltacabtagene autoleucel (cilta-cel) has been given breakthrough therapy designation in China as a potential treatment of relapsed or refractory multiple myeloma. Cilta-cel refers to both LCAR-B38M, the name given to the therapy in China, and JNJ-4528, the name by which it is known outside of China. The…