Belantamab mafodotin, GlaxoSmithKline’s investigational antibody-drug conjugate, continues to show promise when used alone or in combination with other approved therapies to treat people with relapsed or refractory multiple myeloma, according to data from two clinical trials. Findings from both trials — DREAMM-2 (NCT03525678) and DREAMM-6 (NCT03544281) — were presented in two posters at the recent…
Category: Cancer
Keytruda Approved in China as Second-line Therapy for Advanced Esophageal Cancers
The Chinese National Medical Products Administration (NMPA) has approved Keytruda (pembrolizumab) as a second-line therapy for patients with locally advanced or metastatic esophageal squamous cell carcinoma (ESCC) whose tumors have at least 10% of cells producing the PD-L1 protein. With the new approval, Keytruda is now available in China for five indications across three different…
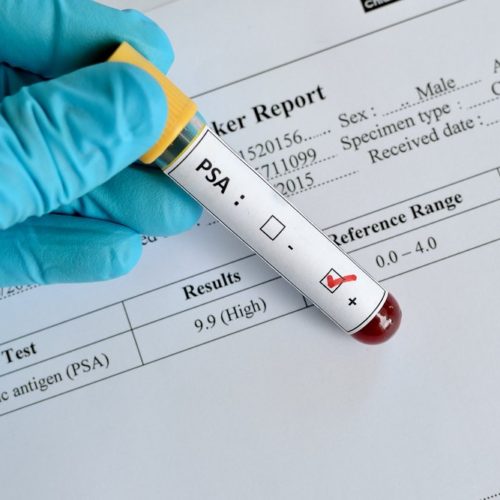
Recommendation Against PSA Routine Screening May Have Contributed to Higher Rates of Advanced PC
The U.S. rates of advanced prostate cancer continued to rise in men 50 and older after the U.S. Preventive Services Task Force (USPSTF) recommended in 2012 against prostate-specific antigen (PSA)-based screening for all men, an American Cancer Society study shows. Notably, the annual increase in men ages 50–74 with the most advanced stage disease after…
Protein Biomarkers to Gauge Cancer Response to Chemo Identified
Investigators using targeted proteomics — a technique to detect proteins of interest — on samples of ovarian cancer found a wide variety of biomarkers that might help predict which chemotherapies patients are more likely to respond to, the precision medicines company mProbe reported. Identifying these tumor biomarkers is crucial not only to spotting potential targets for future therapies, but also…
JNJ-4528 Continues to Show Strong, Durable Responses in Trial
Janssen’s investigational CAR T-cell therapy for multiple myeloma, called JNJ-4528, continues to lead to strong and durable responses in all patients with relapsed or refractory disease who are participating in a Phase 1b/2 trial. These findings were announced in an oral presentation, “Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen…
Imfinzi Plus Chemo Showing 2-year Survival Benefit in Advanced SCLC Study
Adding Imfinzi (durvalumab) to standard chemotherapy as the first-line treatment of adults with extensive-stage small cell lung cancer (SCLC) continues to show survival benefits sustained now for two years, updated findings from the CASPIAN trial show. The ongoing Phase 3 clinical trial (NCT03043872) supported Imfinzi’s recent approval by the U.S. Food and Drug Administration, after demonstrating that this combination…
Pre-Surgical Imfinzi Combo Increases Cancer Eradication Chances in Trial
Treating high-risk, HER2-negative breast cancer patients before surgery with the immunotherapy Imfinzi (durvalumab) plus Lynparza (olaparib) and chemotherapy increases the probability for complete cancer eradication, according to the results from a Phase 2 trial. The results showed the triple combination increased the likelihood of cancer eradication from the breast and lymph nodes when compared with chemotherapy alone.…
Veyonda Aids Radiation Therapy in Reaching Distant Lesions in Late-stage Cancer Trial
Veyonda (idronoxil) given in combination with low-dose radiation therapy was able to shrink metastatic lesions well outside the radiation field in about 27% of men with late-stage metastatic castration-resistant prostate cancer (mCRPC) in the DARRT-1 Phase 1 trial, updated data show. “To our knowledge, this is the first time that anyone has been able to obtain a meaningful abscopal response rate…
Mirvetuximab Soravtansine-Avastin Combo Linked to Promising Responses
When given alongside Avastin (bevacizumab), mirvetuximab soravtansine leads to promising treatment responses in women with recurrent ovarian cancer — particularly those whose tumors contain high levels of the protein folate receptor alpha (FRα), top-line data from a Phase 1b/2 trial shows. The trial findings were presented in “Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug…
Keytruda Approved to Treat Solid Tumors With High Mutational Burden
The U.S. Food and Drug Administration (FDA) has conditionally approved Keytruda (pembrolizumab) for the treatment of pediatric and adult patients with inoperable or metastatic solid tumors with high tumor mutational burden (TMB-H). This is the second time the FDA approved Merck‘s immune checkpoint inhibitor Keytruda for cancer patients with specific genetic features in their tumors rather…