This post was originally published on this site The U.S. Food and Drug Administration (FDA) has approved Janssen‘s Darzalex (daratumumab), used in combination with Revlimid (lenalidomide) and dexamethasone, to treat patients newly diagnosed with multiple myeloma who are ineligible to receive an autologous stem cell transplant (ASCT). Darzalex is an antibody designed to recognize and block…
Category: <span>Cancer</span>
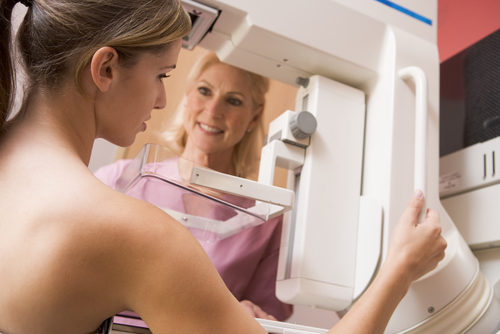
Fujifilm Bus Hits the Road to Offer Breast Cancer Screenings and Promote Early Diagnosis
This post was originally published on this site Fujifilm Medical Systems USA has launched a nationwide effort to enhance access to breast cancer screening and raise awareness about the importance of early disease detection. Called “Aspire to Be Fearless,” the 18-month, 48-state campaign features a mobile mammography bus that will travel around the country to…
PARP Inhibitors Used in ‘One-two Punch’ Strategy to Kill OC Cells in Mouse Models, Study Shows
This post was originally published on this site Researchers have developed a two-step approach that harnesses the aging process of cells, allowing breast and ovarian cancers to be effectively targeted and destroyed in cell and mouse preclinical models. The “one-two punch” strategy consists of first treating cancer cells with PARP inhibitors — an anti-cancer therapy…
Empliciti Combo Prolongs Survival in Difficult-to-Treat Multiple Myeloma, New Phase 2 Trial Data Show
This post was originally published on this site Adding Empliciti (elotuzumab) to a regimen including Pomalyst (pomalidomide) and low-dose dexamethasone extended survival and the time without disease progression in patients with relapsed or refractory multiple myeloma, according to updated results of a Phase 2 study. The study, “Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma:…
X4 Pharmaceuticals, LLS Collaborate to Develop Mavorixafor to Treat Waldenström’s Macroglobulinemia
This post was originally published on this site X4 Pharmaceuticals and The Leukemia & Lymphoma Society (LLS) have joined forces to accelerate the development of X4’s lead product candidate, mavorixafor, for the treatment of Waldenström’s macroglobulinemia, a rare form of non-Hodgkin’s lymphoma. Under LLS’ Therapy Acceleration Program (TAP), X4 will conduct a Phase 1/2 clinical…
Complete Remission Induced in B-cell ALL Patients After Single Dose of FasT CAR-19 Therapy, Preliminary Data Show
This post was originally published on this site A single infusion of an experimental cell therapy called FasT CAR-19 was well-tolerated and induced a complete remission in all B-cell acute lymphoblastic leukemia (B-ALL) patients included in a Phase 1 trial, the therapy’s developer, Gracell Biotechnologies, has announced. All patients had received multiple prior lines of…
Breast Cancer Index Identifies Patients Who Will Benefit From Extended Endocrine Therapy, Data Shows
This post was originally published on this site Biotheranostics‘ Breast Cancer Index can help distinguish which breast cancer patients will derive a benefit from prolonged endocrine therapy — allowing those who don’t to stop treatment and avoid its unpleasant side effects, new data suggests. The findings were shared at the 2019 American Society of Clinical…
Nanostics Launches Pivotal Study to Validate ClarityDX Prostate Diagnostic Test
This post was originally published on this site Nanostics has started a pivotal clinical study in Canada to validate ClarityDX Prostate, a new blood test for the early diagnosis of prostate cancer. The company says the diagnostic tool has the potential to spare many men from unnecessary biopsies and treatments. The study is part of…
Copiktra Induces Promising Clinical Activity in PTCL Patients, Phase 1 Trials Show
This post was originally published on this site Copiktra (duvesilib) shows promising clinical activity in patients with relapsed or refractory peripheral T-cell lymphoma (PTCL), inducing responses in 44% to 57% of patients included in Phase 1 trials. The findings were presented during an oral presentation, “Duvelisib, an oral dual PI3K‐δ,γ inhibitor, efficacy and safety in…
Report Identifies Proteins That Drive Spread in Castration-resistant PC
This post was originally published on this site Inhibiting the protein BRD4 can stop the migration of castration-resistant prostate cancer cells, regardless of the mechanism causing resistance to treatment, new research has shown. This suggests that BRD4 might be a therapeutic target to prevent metastasis (spread) in prostate cancer patients who have become resistant to…